Textbook Question
Use the periodic table to give the electron configuration for each of the following atoms and ions.
(c) Co(V) in CoO43–
(d) Co(IV) in CoF62–

 Verified step by step guidance
Verified step by step guidance
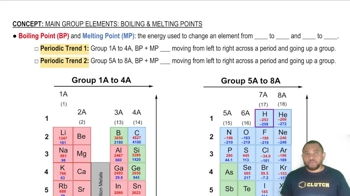

Use the periodic table to give the electron configuration for each of the following atoms and ions.
(c) Co(V) in CoO43–
(d) Co(IV) in CoF62–
Titanium, used to make jet aircraft engines, is much harder than potassium or calcium. Explain.
Briefly account for each of the following observations:
(a) Atomic radii decrease in the order Sc > Ti > V.
(b) Densities increase in the order Ti > V > Cr.
Arrange the following atoms in order of decreasing atomic radius, and account for the trend.
(a) Cr
(b) Ti
(c) Mn
(d) V
What is the lanthanide contraction, and why does it occur?