Ch.21 - Transition Elements and Coordination Chemistry

Chapter 21, Problem 138
In acidic aqueous solution, the complex trans-[Co(en)2Cl2]+ undergoes the following substitution reaction: The reaction is first order in trans-[Co(en)2Cl2]+, and the rate constant at 25 °C is 3.2 × 10⁻⁵ s⁻¹. (a) What is the half-life of the reaction in hours? (b) If the initial concentration of trans-[Co(en)2Cl2]+ is 0.138 M, what is its molarity after a reaction time of 16.5 h? (c) Devise a possible reaction mechanism with a unimolecular rate-determining step.
 Verified step by step guidance
Verified step by step guidance1
Step 1: To find the half-life of a first-order reaction, use the formula: \(t_{1/2} = \frac{0.693}{k}\), where \(k\) is the rate constant.
Step 2: Convert the rate constant from seconds to hours by multiplying by the number of seconds in an hour (3600 s/h).
Step 3: For part (b), use the first-order integrated rate law: \([A] = [A]_0 e^{-kt}\), where \([A]_0\) is the initial concentration, \(k\) is the rate constant, and \(t\) is the time in hours.
Step 4: Substitute the given values into the integrated rate law to find the concentration after 16.5 hours.
Step 5: For part (c), propose a reaction mechanism. A possible mechanism could involve the dissociation of one ligand from the complex to form an intermediate, followed by the substitution of another ligand. The rate-determining step would be the slowest step, likely the initial dissociation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
First-Order Reactions
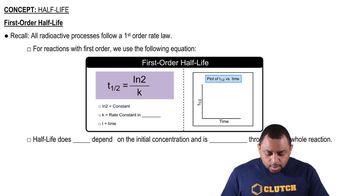
First-order reactions are characterized by a rate that is directly proportional to the concentration of one reactant. In this case, the rate of the reaction involving trans-[Co(en)2Cl2]+ depends solely on its concentration. The half-life of a first-order reaction is constant and can be calculated using the formula t½ = 0.693/k, where k is the rate constant.
Recommended video:
Guided course

First-Order Reactions
Half-Life Calculation
The half-life of a reaction is the time required for the concentration of a reactant to decrease to half of its initial value. For first-order reactions, this value is independent of the initial concentration and can be calculated using the rate constant. Understanding how to calculate half-life is essential for determining how long it takes for a reactant to diminish over time.
Recommended video:
Guided course
First-Order Half-Life
Reaction Mechanism
A reaction mechanism describes the step-by-step sequence of elementary reactions by which overall chemical change occurs. In this context, a unimolecular rate-determining step implies that the rate of the reaction is governed by the transformation of a single reactant species. Proposing a mechanism involves identifying intermediates and the sequence of events leading to product formation, which is crucial for understanding the kinetics of the reaction.
Recommended video:
Guided course

Reaction Mechanism Overview
Related Practice
