Consider the following isomers [Cr(NH3)2Cl4]-.
(a) Label the isomers as cis or trans.
(b) Which isomers are identical, and which are different?
(c) Do any of these isomers exist as enantiomers? Explain.

 Verified step by step guidance
Verified step by step guidance
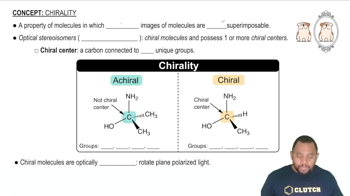
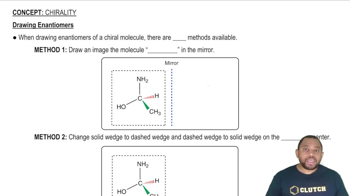
Consider the following isomers [Cr(NH3)2Cl4]-.
(a) Label the isomers as cis or trans.
(b) Which isomers are identical, and which are different?
(c) Do any of these isomers exist as enantiomers? Explain.
Consider the following ethylenediamine complexes.
(a) Which complexes are chiral, and which are achiral?
(b) Draw the enantiomer of each chiral complex.
(c) Which, if any, of the chiral complexes are enantiomers of one another?
Consider the following ethylenediamine complexes.
(a) Which complexes are chiral, and which are achiral?
(b) Draw the enantiomer of each chiral complex.
(c) Which, if any, of the chiral complexes are enantiomers of one another?
Use the periodic table to give the electron configuration for each of the following atoms and ions.
(c) Co(V) in CoO43–
(d) Co(IV) in CoF62–
Titanium, used to make jet aircraft engines, is much harder than potassium or calcium. Explain.