Ch.21 - Transition Elements and Coordination Chemistry

Chapter 21, Problem 142
An alternative to cyanide leaching of gold ores is leaching with thiocyanate ion, which forms a square planar gold(III) complex, Au(SCN)4^3-. (a) If the formation constant for Au(SCN)4^3- is Kf = 10^37, what is the equilibrium concentration of Au^3+ in a 0.050 M solution of Au(SCN)4^3-?
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical equilibrium involved: Au^3+ + 4 SCN^- ⇌ Au(SCN)_4^3-. The formation constant (K_f) for this reaction is given as 10^37.
Write the expression for the formation constant (K_f) in terms of the concentrations: K_f = [Au(SCN)_4^3-] / ([Au^3+][SCN^-]^4).
Assume that the initial concentration of Au(SCN)_4^3- is 0.050 M and that it dissociates slightly to form Au^3+ and SCN^-. Let the change in concentration of Au^3+ be x, so [Au^3+] = x and [SCN^-] = 4x.
Substitute the equilibrium concentrations into the K_f expression: 10^37 = (0.050) / (x * (4x)^4).
Solve the equation for x, which represents the equilibrium concentration of Au^3+. This involves algebraic manipulation and solving a polynomial equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Formation Constant (Kf)
The formation constant, Kf, is a measure of the stability of a complex ion in solution. It quantifies the extent to which reactants form a complex at equilibrium. A higher Kf value indicates a more stable complex, meaning that the equilibrium will favor the formation of the complex over the dissociation into its constituent ions.
Recommended video:
Guided course
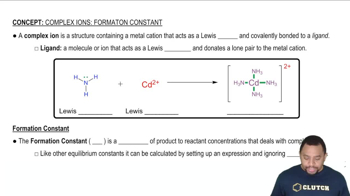
Complex Ions and Formation Constant
Equilibrium Concentration
Equilibrium concentration refers to the concentrations of reactants and products in a chemical reaction at equilibrium. In this context, it is essential to determine the concentration of Au^3+ ions when Au(SCN)4^3- is present in solution. The relationship between the concentrations of the complex and the free ions can be described using the equilibrium expression derived from the formation constant.
Recommended video:
Guided course

Thermal Equilibrium
Square Planar Geometry
Square planar geometry is a molecular shape where four ligands are arranged around a central metal ion in a square plane. This geometry is common for d8 metal ions like Au(III). Understanding the geometry helps in predicting the behavior of the complex in solution, including its stability and reactivity, which are crucial for calculating equilibrium concentrations.
Recommended video:
Guided course
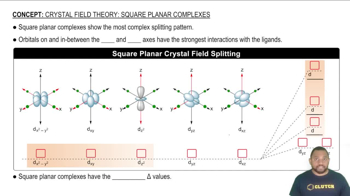
Square planar complexes show the most complex splitting pattern.
Related Practice
