Textbook Question
Iodine has a lower atomic mass than tellurium (126.90 for iodine, 127.60 for tellurium) even though it has a higher atomic number (53 for iodine, 52 for tellurium). Explain.
2
views

 Verified step by step guidance
Verified step by step guidance

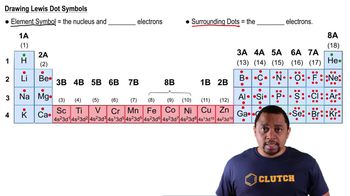
How many protons, neutrons, and electrons are in each of the following atoms? (a)
How many protons, neutrons, and electrons are in each of the following atoms? (b)