Textbook Question
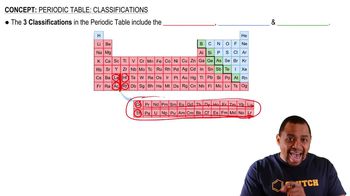
What is the atomic number of the blue element?

 Verified step by step guidance
Verified step by step guidance

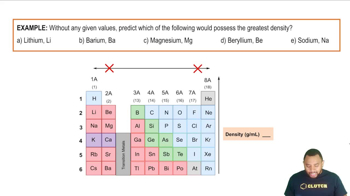
What is the atomic number of the blue element?
What is the group number of the green, blue, and red elements?
Name at least one other element that is chemically similar to the green element.
Identify the three elements indicated on the periodic table, and give the group that they are in.
Classify these elements as metals, nonmetals, or semimetals.
Would you expect these elements to have similar or different chemical reactivity?