Textbook Question
Give systematic names for the following binary compounds: (c) CuO

 Verified step by step guidance
Verified step by step guidance
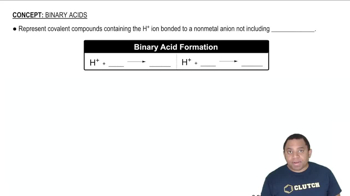


Give systematic names for the following binary compounds: (c) CuO
Lactic acid, a compound found both in sour milk and in tired muscles, has the structure shown. What is its chemical formula?
Give systematic names for the following binary compounds: (a) CsF
Give systematic names for the following binary compounds: (b) K2O
Give systematic names for the following binary compounds: (a) BaS
Give systematic names for the following binary compounds: (b) BeBr2