Textbook Question
At room temperature, a certain element is yellow crystalline solid. It does not conduct electricity and when hit with a hammer, it shatters. Is the element likely to be a metal, a nonmetal, or a semimetal?
3
views

 Verified step by step guidance
Verified step by step guidance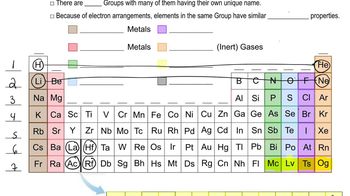

Which type of property—intensive or extensive—does not depend on the amount of substance present?