Textbook Question
Complete and balance the following nuclear equations. (a)

 Verified step by step guidance
Verified step by step guidance
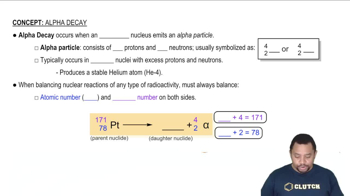


Complete and balance the following nuclear equations. (a)
Complete and balance the following nuclear equations. (b)
What particle is produced in each of the following decay reactions? (a)
What particle is produced in each of the following decay reactions? (b)
What particle is produced in each of the following decay reactions? (c)