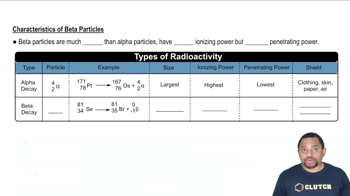
Alpha and Beta Particles
Alpha particles consist of two protons and two neutrons, making them relatively heavy and positively charged. They are emitted during alpha decay, resulting in a decrease in the atomic number by two. Beta particles, on the other hand, are high-energy, high-speed electrons or positrons emitted during beta decay, which changes a neutron into a proton or vice versa, affecting the atomic number by one.

 Verified step by step guidance
Verified step by step guidance