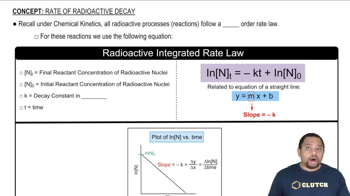
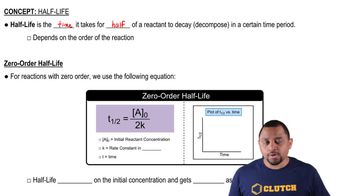
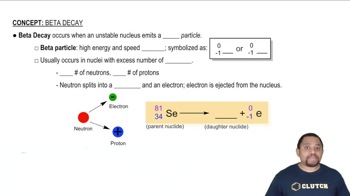
Step 1: Understand that the decay of a radioactive isotope follows first-order kinetics, which can be described by the equation: \( N_t = N_0 e^{-kt} \), where \( N_t \) is the remaining quantity at time \( t \), \( N_0 \) is the initial quantity, \( k \) is the decay constant, and \( t \) is the time elapsed.

 Verified step by step guidance
Verified step by step guidance