Textbook Question
Consider the following table of standard reduction potentials:. Which substance(s) can be reduced by C-?(a) D and B(b) A-(c) D3+ and B2+(d) A

 Verified step by step guidance
Verified step by step guidance
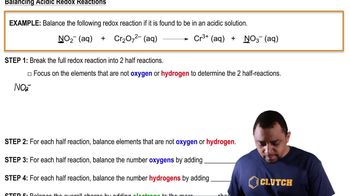
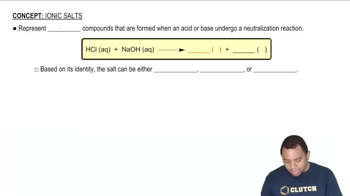
The silver oxide–zinc battery used in watches delivers a voltage of 1.60 V. Calculate the free-energy change (in kilo-joules) for the cell reaction