
Write a balanced equation for the overall cell reaction when a lead storage battery is being charged. Refer to Section 19.10.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
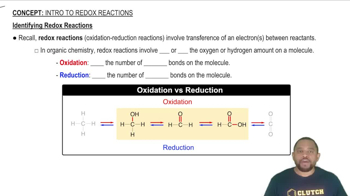
Redox Reactions
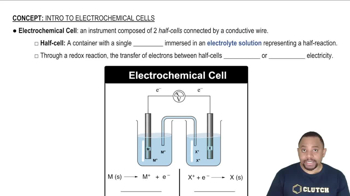
Electrochemical Cells
Balancing Chemical Equations

You are on your dream vacation at the beach when a major storm knocks out the power for days. Your cell phone is dead, and you want to make a battery to charge it. You find the following materials in the beach house. blue stone algaecide for pools, which can be used to make a 1.0 M Cu2+ solution alum in the kitchen, which can be used to make a 1.0 M Al3+ solution aluminum foil, copper wire, and bologna, which can be used as a salt bridge. (b) What voltage can be generated?
You are on your dream vacation at the beach when a major storm knocks out the power for days. Your cell phone is dead, and you want to make a battery to charge it. You find the following materials in the beach house. blue stone algaecide for pools, which can be used to make a 1.0 M Cu2+ solution alum in the kitchen, which can be used to make a 1.0 M Al3+ solution aluminum foil, copper wire, and bologna, which can be used as a salt bridge. (d) An iPhone requires 5.0 V for charging. Can this battery charge the phone? Explain.
A storm has knocked out power to your beach house, and you would like to build a battery from household items to charge your iPhone. You have the following materials. alum in the kitchen, which can be used to make a 1.0 M Al3+ solution bleach, which is a solution that is approximately a 1.0 M in ClO-aluminum foil, a platinum necklace and bologna, which can be used as a salt bridge (a) What are the half-reactions and overall reaction in the battery?
