
What is the reduction potential at 25 °C for the hydrogen electrode in each of the following solutions? The half-reaction is . (c) Pure water.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Reduction Potential

Standard Hydrogen Electrode (SHE)
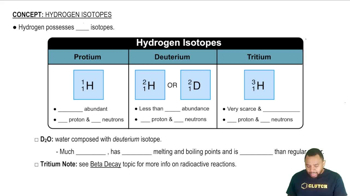
pH and its Effect on Reduction Potential

When suspected drunk drivers are tested with a Breathalyzer, the alcohol (ethanol) in the exhaled breath is oxidized to acetic acid with an acidic solution of potassium dichromate: The color of the solution changes because some of the orange Cr2O72- is converted to the green Cr3+ The Breathalyzer measures the color change and produces a meter reading calibrated in blood alcohol content. (a) What is E° for the reaction if the standard half-cell potential for the reduction of acetic acid to ethanol is 0.058 V?
When suspected drunk drivers are tested with a Breathalyzer, the alcohol (ethanol) in the exhaled breath is oxidized to acetic acid with an acidic solution of potassium dichromate: The color of the solution changes because some of the orange Cr2O72- is converted to the green Cr3+ The Breathalyzer measures the color change and produces a meter reading calibrated in blood alcohol content. (b) What is the value of E for the reaction when the concentrations of ethanol, acetic acid, Cr2O7 are 1.0 M and the pH is 4.00?
