Textbook Question
Write balanced net ionic equations for the following reactions in basic solution. (b)

 Verified step by step guidance
Verified step by step guidance

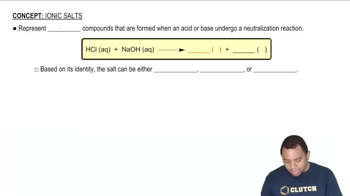
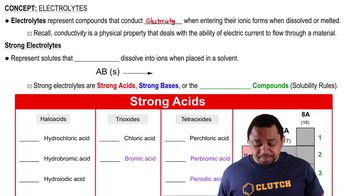
Write balanced net ionic equations for the following reactions in basic solution. (b)
Write balanced net ionic equations for the following reactions in basic solution. (c)