Textbook Question
Write unbalanced oxidation and reduction half-reactions for the following processes. (b) Mn3+(aq) → MnO2(s) + Mn2+(aq)

 Verified step by step guidance
Verified step by step guidance
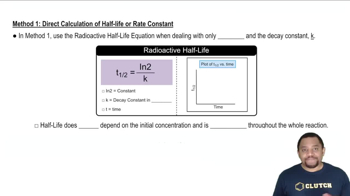

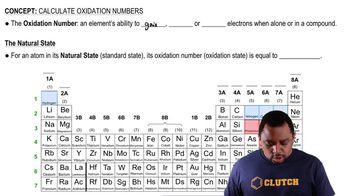
Write unbalanced oxidation and reduction half-reactions for the following processes. (b) Mn3+(aq) → MnO2(s) + Mn2+(aq)
Balance the following half-reactions. (a) (acidic) Cr2O72-(aq) → Cr3+(aq)
Balance the following half-reactions. (b) (basic) CrO42-(aq) → Cr(OH)4-(aq)
Balance the following half-reactions. (d) (basic) ClO-(aq) → Cl-(aq)
Balance the following half-reactions. (a) (acidic) VO2+(aq) → V3+(aq)
Balance the following half-reactions. (b) (basic) Ni(OH)2(s) → Ni2O3(s)