Textbook Question
Balance the following half-reactions. (d) (basic) ClO-(aq) → Cl-(aq)

 Verified step by step guidance
Verified step by step guidance
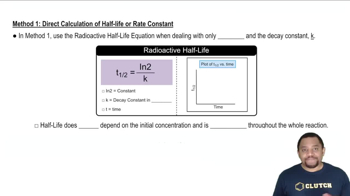

Balance the following half-reactions. (d) (basic) ClO-(aq) → Cl-(aq)
Balance the following half-reactions. (a) (acidic) VO2+(aq) → V3+(aq)
Balance the following half-reactions. (b) (basic) Ni(OH)2(s) → Ni2O3(s)
Balance the following half-reactions. (d) (basic) Br2(aq) → BrO3-(aq)
Write balanced net ionic equations for the following reactions in acidic solution. (b) TeO2(s) + Cr2+(aq) → Te(s) + Cr3+(aq)
Write balanced net ionic equations for the following reactions in acidic solution. (c) I-(aq) + IO3-(aq) → I3-(aq)