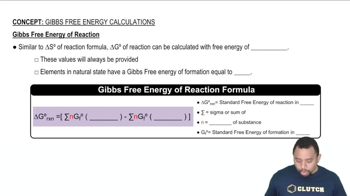
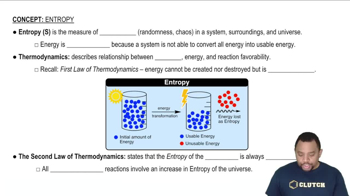
Textbook Question
Trouton's rule says that the ratio of the molar heat of vaporization of a liquid to its normal boiling point (in kelvin) is approximately the same for all liquids: ∆Hvap/Tbp ≈ 88 J/(K*mol) (a) Check the reliability of Trouton's rule for the liquids listed in the following table.
1
views