
 McMurry 8th Edition
McMurry 8th Edition Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium
Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium Problem 141a
Problem 141aConsider the unbalanced equation: (a) Balance the equation for this reaction in basic solution.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
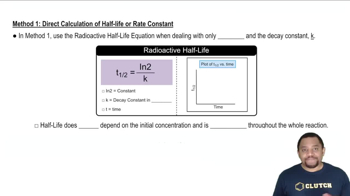
Key Concepts
Balancing Chemical Equations

Basic Solution

Half-Reaction Method
The lead storage battery uses the reaction: (b) Calculate ∆G for this reaction on a cold winter's day (10 °F) in a battery that has run down to the point where the sulfuric acid concentration is only 0.100 M.
Consider the unbalanced equation: (b) Use the data in Appendix B and ΔG°f for IO3-(aq)= -128.0 kJ/mol to calculate ΔG° for the reaction at 25 °C.
Consider the unbalanced equation: I2(s) → I-(aq) + IO3-(aq) (d) What pH is required for the reaction to be at equilibrium at 25°C when [I-] = 0.10M and [IO3-] = 0.50 M?
Methanol (CH3OH) is made industrially in two steps from CO and H2. It is so cheap to make that it is being considered for use as a precursor to hydrocarbon fuels, such as methane (CH4):
Step 1. CO(g) + 2 H2(g) S CH3OH(l) ΔS° = - 332 J/K
Step 2. CH3OH1l2 → CH4(g) + 1/2 O2(g) ΔS° = 162 J/K
(k) Calculate an overall ΔG°, ΔH°, and ΔS° for the formation of CH4 from CO and H2.