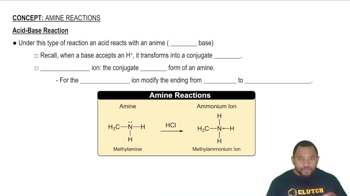
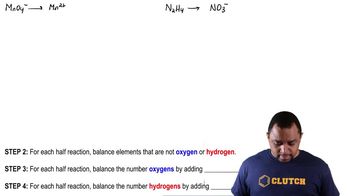
Textbook Question
Use the values of of ∆G°f in Appendix B to calculate the stan-dard free-energy change for the synthesis of dichloroethane from ethylene and chlorine:C2H41g2 + Cl21g2S CH2ClCH2Cl1l2Is it possible to synthesize dichloroethane from gaseous C2H4 and Cl2, each at 25 °C and 1 atm pressure?