Textbook Question
If the following solutions are mixed, is the resulting solution acidic, basic, or neutral?(a) 50.0 mL of 0.100 M HBr and 30.0 mL of 0.200 M KOH

 Verified step by step guidance
Verified step by step guidance

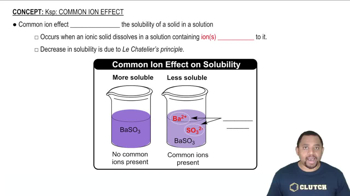
Use Le Châtelier's principle to predict whether the solubility of BaF2 will increase, decrease, or remain the same on addition of each of the following substances. (a) HCl
Use Le Châtelier's principle to predict whether the solubility of BaF2 will increase, decrease, or remain the same on addition of each of the following substances. (b) KF
Use Le Châtelier's principle to predict whether the solubility of BaF2 will increase, decrease, or remain the same on addition of each of the following substances. (c) NaNO3