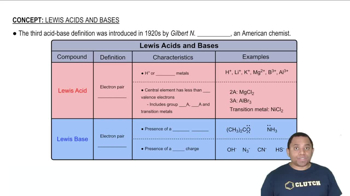
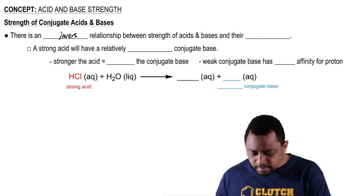
The following pictures represent solutions at various stages in the titration of sulfuric acid H2A (A2- = SO4 2-) with aqueous NaOH. (Na+ ions and water molecules have been omitted for clarity.)
. (b) Which solution has the highest pH? Draw a picture that represents the solution prior to addition of any NaOH.