Consider the titration of 50.0 mL of a 0.100 M solution of the protonated form of the amino acid alanine (H2A+: Ka1 = 4.6 × 10–3, Ka2 = 2.0 × 10–10) with 0.100 M NaOH. Calculate the pH after the addition of each of the following volumes of base. (b) 25.0 mL (c) 50.0 mL

Consider the titration of 25.0 mL of 0.0200 M H2CO3 with 0.0250 M KOH. Calculate the pH after the addition of each of the following volumes of base: (a) 10.0 mL, (b) 20.0 mL, (c) 30.0 mL, (d) 40.0 mL.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Titration

pH and Acid-Base Chemistry
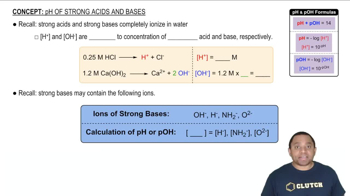
Buffer Solutions

Consider the titration of 50.0 mL of a 0.100 M solution of the protonated form of the amino acid alanine (H2A+: Ka1 = 4.6 × 10–3, Ka2 = 2.0 × 10–10) with 0.100 M NaOH. Calculate the pH after the addition of each of the following volumes of base. (d) 75.0 mL
Consider the titration of 50.0 mL of a 0.100 M solution of the protonated form of the amino acid alanine (H2A+: Ka1 = 4.6 × 10–3, Ka2 = 2.0 × 10–10) with 0.100 M NaOH. Calculate the pH after the addition of each of the following volumes of base. (e) 100.0 mL
Consider the titration of 50.0 mL of 1.00 M H3PO4 with 1.00 M KOH. Calculate the pH after the addition of each of the following volumes of base. (a) 25.0 mL (c) 75.0 mL
Consider the titration of 50.0 mL of 1.00 M H3PO4 with 1.00 M KOH. Calculate the pH after the addition of each of the following volumes of base. (b) 50.0 mL (d) 100.0 mL
