
A saturated solution of an ionic salt MX exhibits an osmotic pressure of 74.4 mm Hg at 25 °C. Assuming that MX is completely dissociated in solution, what is the value of its Ksp?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
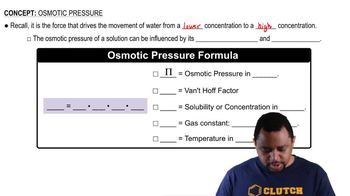
Osmotic Pressure
Dissociation of Ionic Compounds

Solubility Product Constant (Ksp)

A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equivalence point was reached after 126.4 mL of base. (c) What percent of the HCl is neutralized at the first equivalence point?
A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equivalence point was reached after 126.4 mL of base. (f) What indicators would you select to signal the equivalence points?
In qualitative analysis, Ca2+ and Ba2+ are seperated from Na+, K+, Mg2+ by adding aqueous (NH4)2CO3 to a solution that also contains aqueous NH3 (Figure 17.18). Assume that the concentrations after mixing are 0.080 M (NH4)2CO3 and 0.16 M NH3. (a) List all the Bronsted-Lowry acids and bases present initially, and identify the principal reaction.
