Textbook Question
Calculate the pH of 0.375 L of a 0.18 M acetic acid–0.29 M sodium acetate buffer before and after the addition of (a) 0.0060 mol of KOH. Assume that the volume remains constant.

 Verified step by step guidance
Verified step by step guidance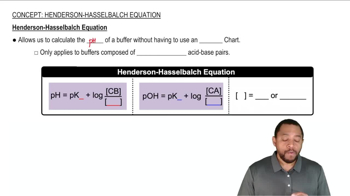
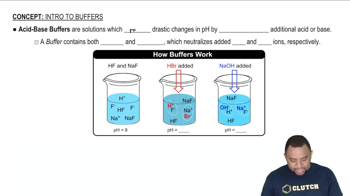
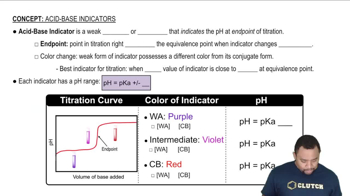
Calculate the pH of 0.375 L of a 0.18 M acetic acid–0.29 M sodium acetate buffer before and after the addition of (a) 0.0060 mol of KOH. Assume that the volume remains constant.
Calculate the pH of 0.375 L of a 0.18 M acetic acid–0.29 M sodium acetate buffer before and after the addition of (b) 0.0060 mol of HBr. Assume that the volume remains constant.