Textbook Question
The hydronium ion H3O+ is the strongest acid that can exist in aqueous solution because stronger acids dissociate by transferring a proton to water. What is the strongest base that can exist in aqueous solution?

 Verified step by step guidance
Verified step by step guidance
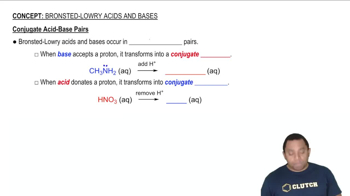
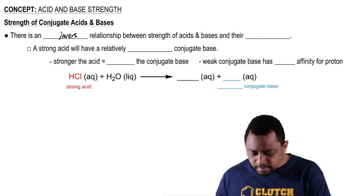

Arrange each group of compounds in order of increasing acid strength. Explain your reasoning. (a) HCl, H2S, PH3