Acid-Base Equilibrium
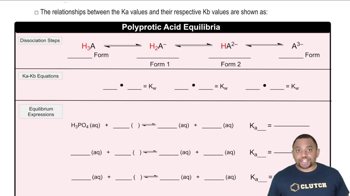
Acid-base equilibrium involves the balance between the concentrations of acids and their conjugate bases in a solution. The dissociation of benzoic acid (C6H5COOH) into its ions establishes this equilibrium. The equilibrium constant, Ka, quantifies the strength of the acid, and is essential for calculating the concentrations of all species in the solution, including H3O+ and OH-.

 Verified step by step guidance
Verified step by step guidance