pH Calculation
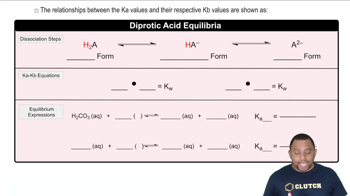
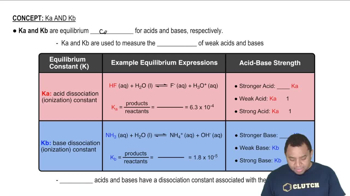
pH is a measure of the hydrogen ion concentration in a solution, calculated using the formula pH = -log[H⁺]. For strong acids, the pH can often be determined directly from the concentration of the acid, while for weak acids, it requires consideration of the dissociation constants. In the case of diprotic acids like selenic acid, both dissociation steps must be accounted for to accurately calculate the pH and the concentrations of all species present.

 Verified step by step guidance
Verified step by step guidance