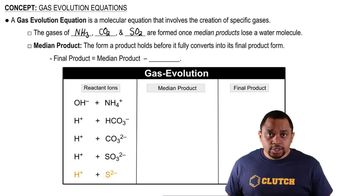
Textbook Question
The hydrated cation M1H2O26 3 + has Ka = 10-4, and the acid HA has Ka = 10-5. Identify the principal reaction in an aqueous solution of each of the following salts, and classify each solution as acidic, basic, or neutral. (a) NaA

 Verified step by step guidance
Verified step by step guidance