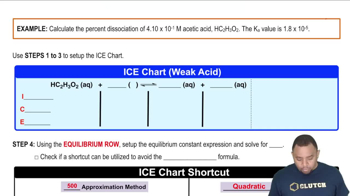
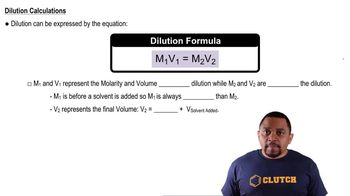
Textbook Question
During a certain time period, 4.0 million tons of SO2 were released into the atmosphere and subsequently oxidized to SO3. As explained in the Inquiry, the acid rain produced when the SO3 dissolves in water can damage marble statues: CaCO3(s) + H2SO4(aq) → CaSO4(aq) + CO2(g) + H2O(l) (a) How many 500 pound marble statues could be damaged by the acid rain? (Assume that the statues are pure CaCO3 and that a statue is damaged when 3.0% of its mass is dissolved.)