The F-F bond in F2 is relatively weak because the lone pairs of electrons on one F atom repel the lone pairs on the other F atom; Kp = 7.83 at 1500 K for the reaction F2(g) ⇌ 2 F(g). (a) If the equilibrium partial pressure of F2 molecules at 1500 K is 0.200 atm, what is the equilibrium partial pressure of F atoms in atm?

When 0.500 mol of N2O4 is placed in a 4.00-L reaction vessel and heated at 400 K, 79.3% of the N2O4 decomposes to NO2. (a) Calculate Kc and Kp at 400 K for the reaction N2O4(g) ↔ 2 NO2(g).
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Equilibrium Constant (Kc)

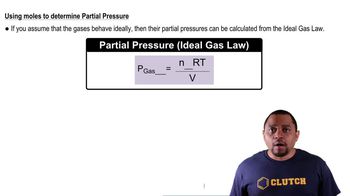
Partial Pressure and Kp
Stoichiometry and Reaction Extent

The F-F bond in F2 is relatively weak because the lone pairs of electrons on one F atom repel the lone pairs on the other F atom; Kp = 7.83 at 1500 K for the reaction F2(g) ⇌ 2 F(g). (b) What fraction of the F2 molecules dissociate at 1500 K?
The F-F bond in F2 is relatively weak because the lone pairs of electrons on one F atom repel the lone pairs on the other F atom; Kp = 7.83 at 1500 K for the reaction F2(g) ⇌ 2 F(g). (c) Why is the F-F bond in F2 weaker than the Cl-Cl bond in Cl2?
The equilibrium constant Kc for the gas-phase thermal decomposition of cyclopropane to propene is 1.0 ⨉105 at 500 K:
(a) What is the value of Kp at 500 K?
The equilibrium constant Kc for the gas-phase thermal decomposition of cyclopropane to propene is 1.0 * 105 at 500 K:
(c) Can you alter the ratio of the two concentrations at equilibrium by adding cyclopropane or by decreasing the volume of the container? Explain.
