Textbook Question
The following pictures represent the composition of the equi- librium mixture for the reaction A + B ∆ AB at 300 K and at 400 K. Is the reaction exothermic or endothermic? Explain using Le Châtelier's principle.

 Verified step by step guidance
Verified step by step guidance

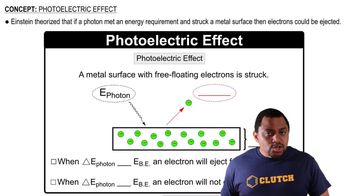
The following pictures represent the initial and equilibrium states for the exothermic decomposition of gaseous A mol- ecules (red) to give gaseous B molecules (blue). (a) Write a balanced chemical equation for the reaction.
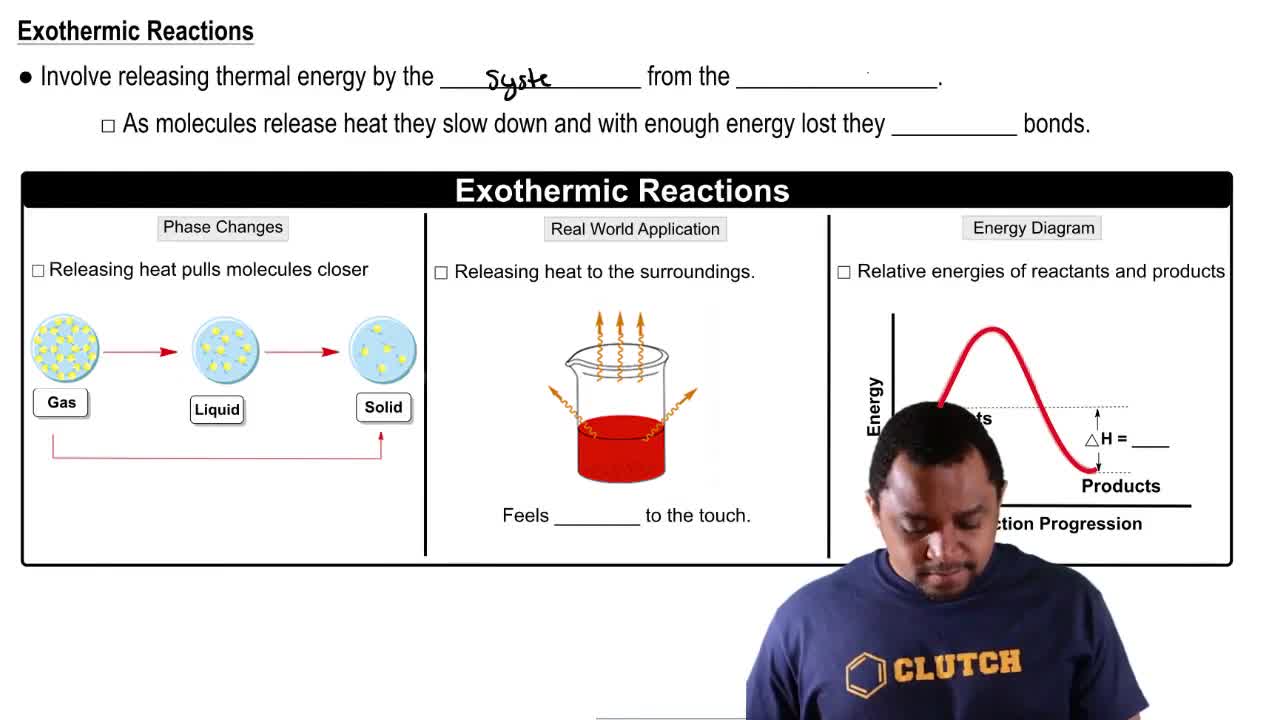
The following pictures represent the initial and equilibrium states for the exothermic reaction of solid A (red) with gas- eous B2 (blue) to give gaseous AB. (a) Write a balanced chemical equation for the reaction.