
The decomposition of solid ammonium carbamate, (NH4)2NH2CO2, to gaseous ammonia and carbon dioxide is an endothermic reaction: (NH4)2NH2CO2(s) ⇌ 2 NH3(g) + CO2(g). (a) When solid (NH4)2NH2CO2 is introduced into an evacuated flask at 25 °C, the total pressure of gas at equilibrium is 0.116 atm. What is the value of Kp at 25 °C?(b) Given that the decomposition reaction is at equilibrium, how would the following changes affect the total quantity of NH3 in the flask once equilibrium is reestablished? (i) Adding CO2 (ii) Removing CO2 (iii) Adding (NH4)2NH2CO2 (iv) Increasing the total volume (v) Adding neon (vi) Increasing the temperature
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Equilibrium Constant (Kp)

Le Chatelier's Principle

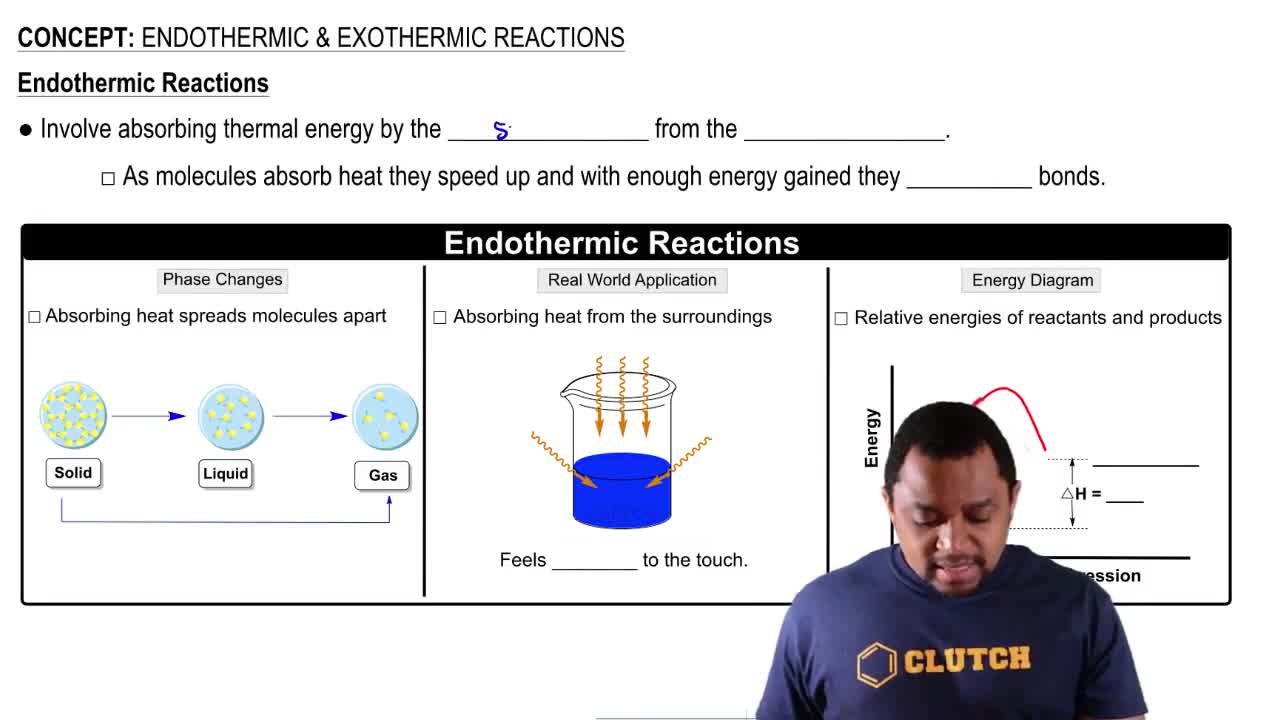
Endothermic Reactions
Calculate the value of the equilibrium constant at 427 °C for the reaction
Na O1s2 + 1>2 O 1g2 ∆ Na O 1s2
given the following equilibrium constants at 427 °C.
Na2O1s2 ∆ 2 Na1l2 + 1>2 O21g2 Kc = 2 * 10-25 Na O 1s2 ∆ 2 Na1l2 + O 1g2 K = 5 * 10-29
Calculate the value of the equilibrium constant for the reaction 4 NH31g2 + 3 O21g2 ∆ 2 N21g2 + 6 H2O1g2
given the following equilibrium constants at a certain temperature.
2 H21g2 + O21g2 ∆ 2 H2O1g2 Kc = 3.2 * 1081
N21g2 + 3 H21g2 ∆ 2 NH31g2 Kc = 3.5 * 108
The reaction
2 PH31g2 + As21g2 ∆ 2 AsH31g2 + P21g2
has Kp = 2.9 * 10-5 at 873 K. At the same temperature,
what is Kp for each of the following reactions?
(a) 2 AsH31g2 + P21g2 ∆ 2 PH31g2 + As21g2
If Kc = 7.5×10−9 at 1000 K for the reaction N2(g) + O2(g) → 2 NO(g), give the value of Kc at 1000 K for the reaction
(a) 2 NO(g) → N2(g) + O2(g)
