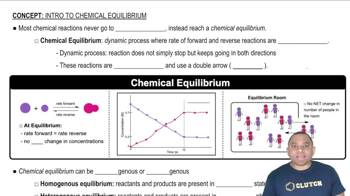
Textbook Question
Consider the interconversion of A molecules (red spheres) and B molecules (blue spheres) according to the reaction A ∆ B. Each of the series of pictures at the right represents a separate experiment in which time increases from left to right:(b) What is the value of the equilibrium constant Kc for the reaction A ∆ B?