Textbook Question
Consider the following equilibrium: Ag+ (aq) + Cl-(aq) → AgCl(s) Use Le Châtelier's principle to predict how the amount of solid silver chloride will change when the equilibrium is disturbed by: (a) Adding NaCl (b) Adding AgNO3

 Verified step by step guidance
Verified step by step guidance


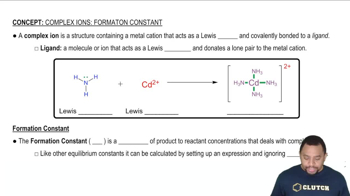
Consider the following equilibrium: Ag+ (aq) + Cl-(aq) → AgCl(s) Use Le Châtelier's principle to predict how the amount of solid silver chloride will change when the equilibrium is disturbed by: (a) Adding NaCl (b) Adding AgNO3
Consider the following equilibrium: Ag+(aq) + Cl-(aq) ⇌ AgCl(s) Use Le Châtelier's principle to predict how the amount of solid silver chloride will change when the equilibrium is disturbed by: (d) Removing Cl-; also account for the change using the reaction quotient Qc