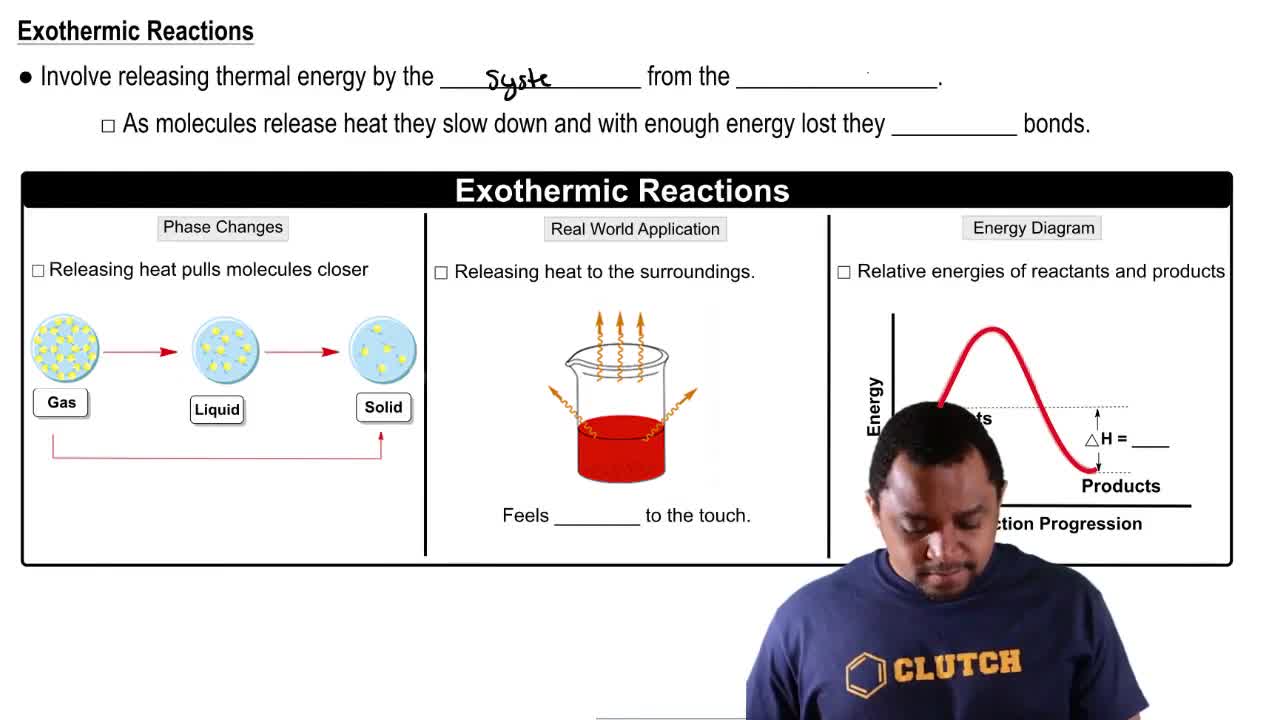
Textbook Question
For the water–gas shift reaction CO1g2 + H2O1g2 ∆ CO21g2 + H21g2, ΔH° = - 41.2 kJ does the amount of H2 in an equilibrium mixture increase or decrease when the temperature is increased? How does Kc change when the temperature is decreased? Justify your answers using Le Châtelier's principle.