Textbook Question
The following experimental data were obtained in a studyof the reaction 2 HI1g2S H21g2 + I21g2. Predict the concentrationof HI that would give a rate of 1.0 * 10-5 M>sat 650 K.

 Verified step by step guidance
Verified step by step guidance
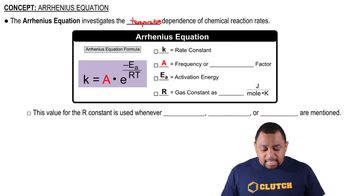

Values of Ea = 6.3 kJ/mol and A = 6.0⨉108/(M s) have been measured for the bimolecular reaction: NO(g) + F2(g) → NOF(g) + F(g) (a) Calculate the rate constant at 25 °C.
The rate constant for the first-order decomposition of gaseous N2O5 to NO2 and O2 is 1.7 * 10-3 s-1 at 55 °C. (a) If 2.70 g of gaseous N2O5 is introduced into an evacuated 2.00 L container maintained at a constant temperature of 55 °C, what is the total pressure in the container after a reaction time of 13.0 minutes?