Textbook Question
Consider the following concentration–time data for the decomposition reaction AB → A + B.
(a) Determine the order of the reaction and the value of the rate constant.

 Verified step by step guidance
Verified step by step guidance
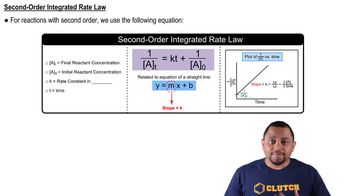
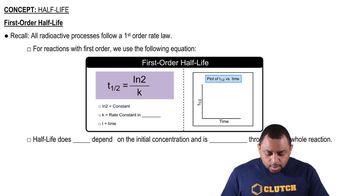
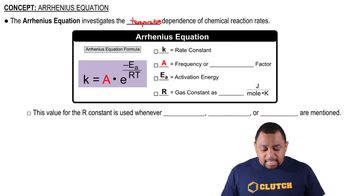
Consider the following concentration–time data for the decomposition reaction AB → A + B.
(a) Determine the order of the reaction and the value of the rate constant.
Consider the following concentration–time data for the decomposition reaction AB → A + B.
(b) What is the molarity of AB after a reaction time of 192 min?