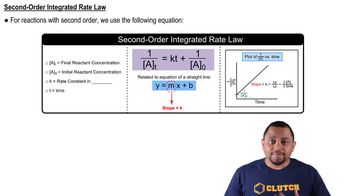
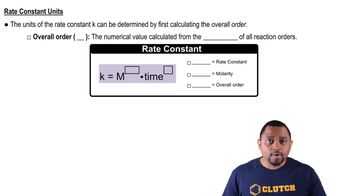
Textbook Question
Use the following equation and graph to answer questions 1 and 2.Hydrogen iodide decomposes at 410 °C, according the reaction:2 HI1g2¡H21g2 + I21g2The graph shows how the concentration of HI changes over time.What is the average rate of loss of HI over the time period0–40 s (LO 14.1)(a) 7.5 * 10-3 M>s (b) 4.8 * 10-3 M>s(c) 3.0 * 10-2 M>s (d) 3.5 * 10-3 M>s
1
views