Consider the following concentration–time data for the reaction of iodide ion and hypochlorite ion (OCl-). The products are chloride ion and hypoiodite ion (OI-).
(a) Write a balanced equation for the reaction.

 Verified step by step guidance
Verified step by step guidance


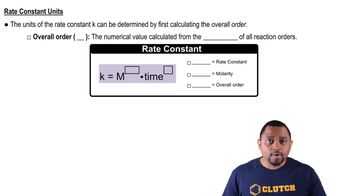
Consider the following concentration–time data for the reaction of iodide ion and hypochlorite ion (OCl-). The products are chloride ion and hypoiodite ion (OI-).
(a) Write a balanced equation for the reaction.
Consider the following concentration–time data for the reaction of iodide ion and hypochlorite ion (OCl-). The products are chloride ion and hypoiodite ion (OI-).
(b) Determine the rate law, and calculate the value of the rate constant.
Consider the reversible, first-order interconversion of two molecules A and B: where kf = 3.0⨉10-3 s-1 is the rate constant for the forward reaction and kr = 1.0⨉10-3 s-1 is the rate constant for the reverse reaction. We'll see in Chapter 15 that a reaction does not go to completion but instead reaches a state of equilibrium with comparable concentrations of reactants and products if the rate constants kf and kr have comparable values.
(a) What are the rate laws for the forward and reverse reactions?
Consider the reversible, first-order interconversion of two molecules A and B: where kf = 3.0⨉10-3 s-1 is the rate constant for the forward reaction and kr = 1.0⨉10-3 s-1 is the rate constant for the reverse reaction. We'll see in Chapter 15 that a reaction does not go to completion but instead reaches a state of equilibrium with comparable concentrations of reactants and products if the rate constants kf and kr have comparable values.
(b) Draw a qualitative graph that shows how the rates of the forward and reverse reactions vary with time.
Consider the reversible, first-order interconversion of two molecules A and B: where kf = 3.0⨉10-3 s-1 is the rate constant for the forward reaction and kr = 1.0⨉10-3 s-1 is the rate constant for the reverse reaction. We'll see in Chapter 15 that a reaction does not go to completion but instead reaches a state of equilibrium with comparable concentrations of reactants and products if the rate constants kf and kr have comparable values.
(c) What are the relative concentrations of B and A when the rates of the forward and reverse reactions become equal?