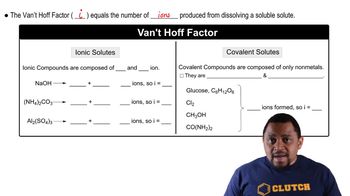
Identify the formula for vapor pressure depression: \( \Delta P = i \cdot m \cdot K_f \), where \( \Delta P \) is the vapor pressure depression, \( i \) is the van’t Hoff factor, \( m \) is the molality, and \( K_f \) is the cryoscopic constant. However, since we are dealing with vapor pressure, we use \( \Delta P = i \cdot m \cdot P^0 \), where \( P^0 \) is the vapor pressure of the pure solvent.

 Verified step by step guidance
Verified step by step guidance