Textbook Question
Which of the following solutions is more concentrated? (a) 0.500 M KCl or 0.500 mass % KCl in water
1
views

 Verified step by step guidance
Verified step by step guidance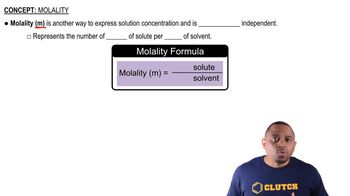
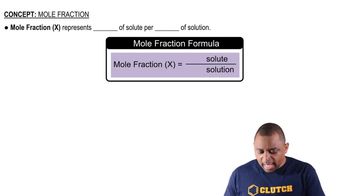

Which of the following solutions is more concentrated? (a) 0.500 M KCl or 0.500 mass % KCl in water