Textbook Question
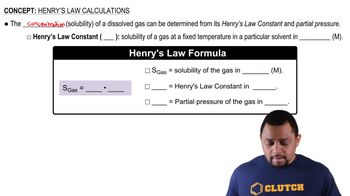
At an altitude of 10,000 ft, the partial pressure of oxygen in the lungs is about 68 mm Hg. What is the concentration in mg/L of dissolved O2 in blood (or water) at this partial pres- sure and a normal body temperature of 37 °C? The solubil- ity of O2 in water at 37 °C and 1 atm partial pressure is 1.93 * 10-3 mol>L.