Treatment of 1.385 g of an unknown metal M with an excess of aqueous HCl evolved a gas that was found to have a volume of 382.6 mL at 20.0 °C and 755 mm Hg pressure. Heating the reaction mixture to evaporate the water and remaining HCl then gave a white crystalline compound, MClx. After dis- solving the compound in 25.0 g of water, the melting point of the resulting solution was - 3.53 °C. (e) What are the formula and molecular weight of MClx?

Combustion analysis of a 36.72-mg sample of the male hormone testosterone gave 106.43 mg CO2 and 32.10 mg H2O as the only combustion products. When 5.00 mg of testosterone was dissolved in 15.0 mL of a suitable solvent at 25 °C, an osmotic pressure of 21.5 mm Hg was measured. What is the molecular formula of testosterone?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Combustion Analysis

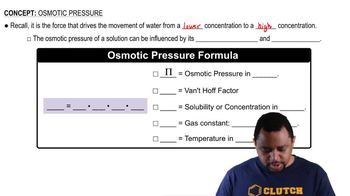
Osmotic Pressure
Molecular Formula

Treatment of 1.385 g of an unknown metal M with an excess of aqueous HCl evolved a gas that was found to have a volume of 382.6 mL at 20.0 °C and 755 mm Hg pressure. Heating the reaction mixture to evaporate the water and remaining HCl then gave a white crystalline compound, MClx. After dis- solving the compound in 25.0 g of water, the melting point of the resulting solution was - 3.53 °C. (f) What is the identity of the metal M?
A compound that contains only C and H was burned in excess O2 to give CO2 and H2O. When 0.270 g of the com- pound was burned, the amount of CO2 formed reacted completely with 20.0 mL of 2.00 M NaOH solution according to the equation 2 OH-1aq2 + CO21g2 S CO 2- 1aq2 + H2O1l2 When 0.270 g of the compound was dissolved in 50.0 g of camphor, the resulting solution had a freezing point of 177.9 °C. [#Pure camphor freezes at 179.8 °C and has Kf = 37.7 1°C kg2>mol.] (a) What is the empirical formula of the compound?
