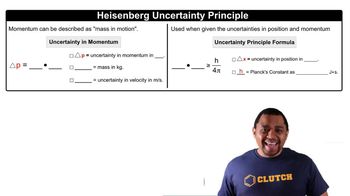
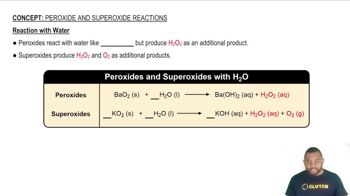
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between molecules. They include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Understanding these forces is crucial for predicting the behavior of substances in different environments, such as solubility in solvents.

 Verified step by step guidance
Verified step by step guidance