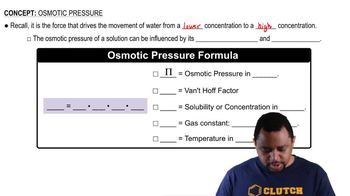
Textbook Question
Human blood gives rise to an osmotic pressure of approxi-mately 7.7 atm at body temperature, 37.0 °C. What must the molarity of an intravenous glucose solution be to give rise to the same osmotic pressure as blood?

 Verified step by step guidance
Verified step by step guidance