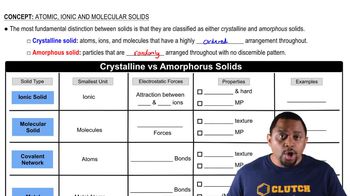
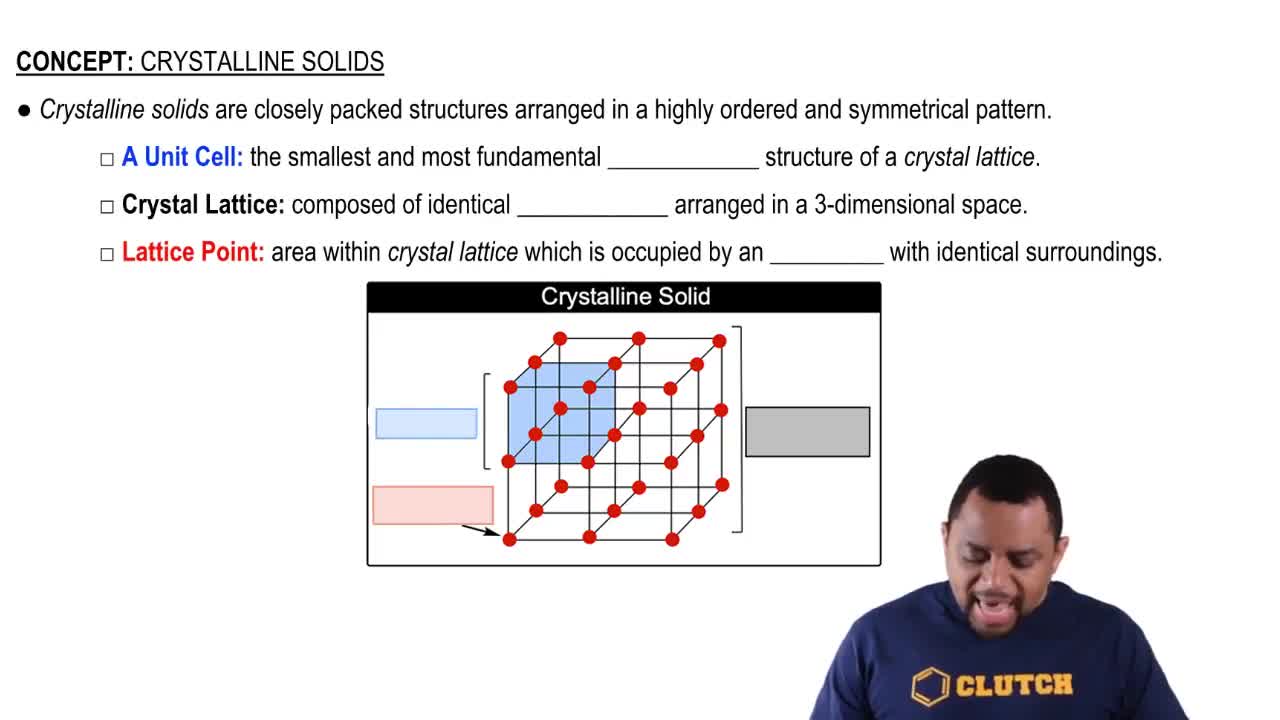
Properties of Metals and Nonmetals
Metals, such as gold (Au), are characterized by their ability to conduct electricity and heat, malleability, and ductility due to the delocalized electrons in their structure. Nonmetals, including substances like quartz and rubber, exhibit different properties, such as brittleness and poor conductivity. Understanding these properties helps in identifying which substances fit the descriptions provided in the question.

 Verified step by step guidance
Verified step by step guidance