Textbook Question
What is the coordination environment of the K+ ions in thefullerene-based superconductor K3C60?

 Verified step by step guidance
Verified step by step guidance
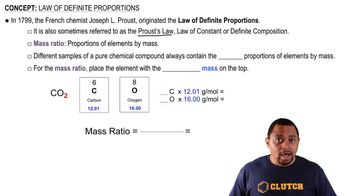
The YBa2Cu3O7 superconductor can be synthesized by the solgel method from a stoichiometric mixture of metal ethoxides followed by heating in oxygen. How many grams of Y(OCH2CH3)3 and how many grams of Ba(OCH2CH3)2 are required to react with 75.4 g of Cu(OCH2CH3)2 and an excess of water? Assuming a 100% yield, how many grams of YBa2Cu3O7 are obtained?