Textbook Question
What is the mole fraction of each gas in the mixture described in Problem 10.83?

 Verified step by step guidance
Verified step by step guidance

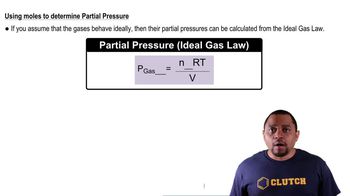
A mixture of 14.2 g of H2 and 36.7 g of Ar is placed in a 100.0-L container at 290 K. (a) What is the partial pressure of H2 in atmospheres?
Chlorine gas was first prepared in 1774 by the oxidation of NaCl with MnO2: 2 NaCl(s) + 2 H2SO4(l) + MnO2(s) → Na2SO4(s) + MnSO4(s) + 2 H2O(g) + Cl2(g) Assume that the gas produced is saturated with water vapor at a partial pressure of 28.7 mm Hg and that it has a volume of 0.597 L at 27 °C and 755 mm Hg pressure. (a) What is the mole fraction of Cl2 in the gas?