Textbook Question
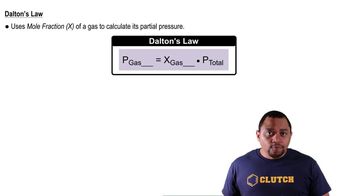
Natural gas is a mixture of many substances, primarily CH4, C2H6, C3Hg, and C4H10. Assuming that the total pressure of the gases is 1.48 atm and that their mole ratio is 94:4.0:1.5:0.50, calculate the partial pressure in atmospheres of each gas.